
Atomic fission uranium free#
The free neutrons can go on to promote additional fissions in a chain reaction. Several neutrons accompany that process, since neither of the smaller resulting nuclei needed so many neutrons to find a home on the nuclear stability curve. However, when it comes to nuclear fission, uranium-235 can break into significantly large pieces, like the barium and krypton. The same is true of the most abundant isotope of uranium, uranium-238. Thorium by and large undergoes radioactive decay to become the smaller, stable nucleus of lead. The less-common uranium-235, with a half-life of about 700 million years, is one such nucleus. A fissile nucleus can be split into two or more smaller nuclei of substantial size, usually because of a collision with a neutron. A Fissile NucleusĬertain isotopes of these heavier actinoids are what scientists call ‘fissile’. But with uranium and elements to its right, there is a short-cut available, an alternative path to stability-the process of fission. The loss of two protons and two neutrons per alpha particle, successively, decreases the mass of the remaining nucleus on its way to a stable end point. Most radioactive isotopes, which are lighter than uranium, emit alpha particles as part of their decay chain. Only when enough fissile material is packed into a small enough volume can we reach the condition where neutron flux is sufficiently high to cause a runaway chain reaction.
In both uranium and plutonium, a specific amount of weapons-grade material is necessary to achieve what is called ‘criticality’. (Image: adison pangchai/Shutterstock) The Decay Chain Plutonium-239 was used in the ‘Fat Man’ bomb dropped on Nagasaki-and a month earlier in a bomb test that took place in Alamogordo, New Mexico. Uranium-235 was used in the ‘Little Boy’ bomb dropped on Hiroshima.
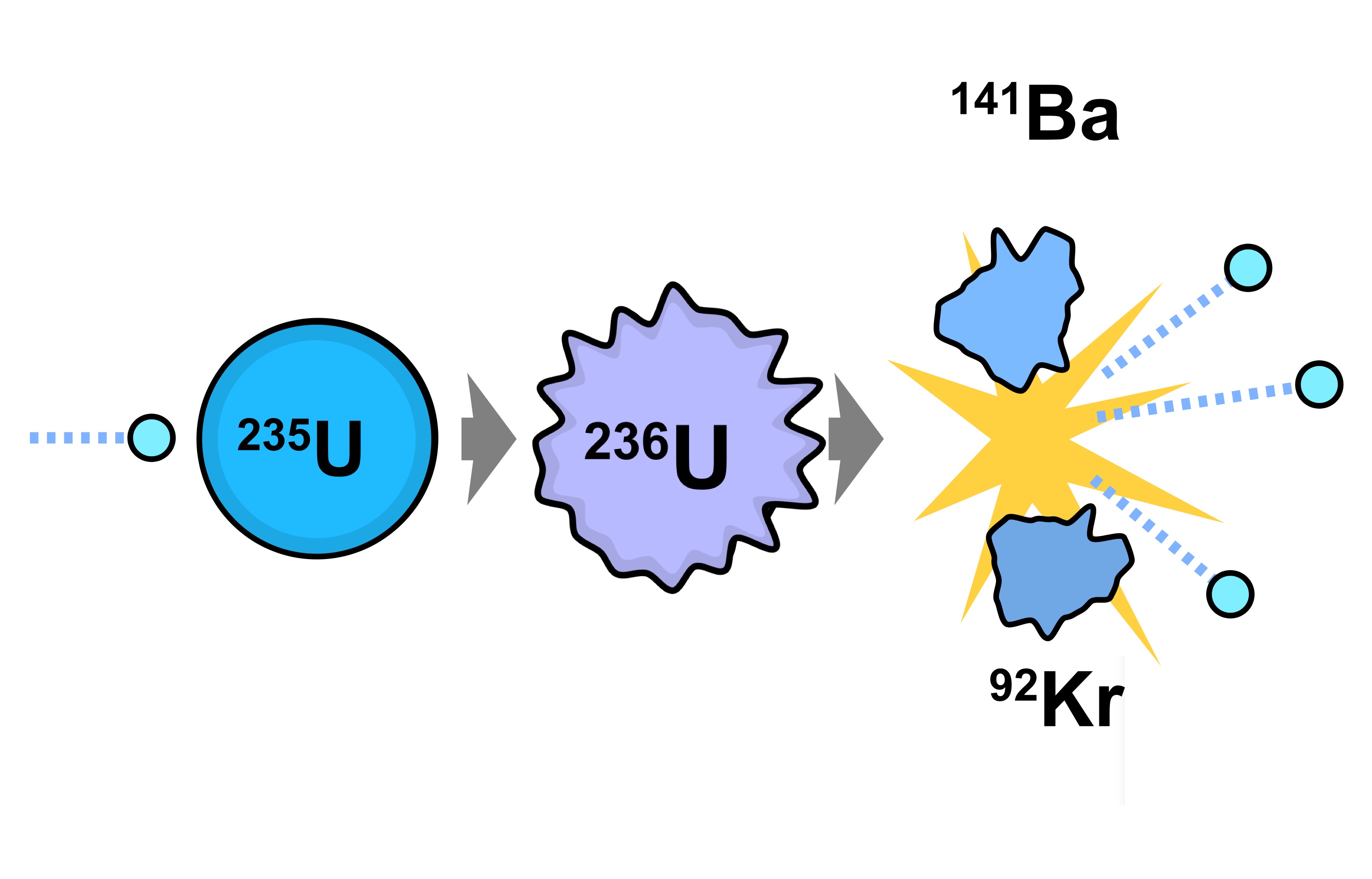
Davis Jr., Georgetown University The first and only nuclear devices ever deployed in wartime used extremely concentrated samples of fissile nuclei obtained through nuclear fission.


 0 kommentar(er)
0 kommentar(er)
